潘赛勇课题组介绍
|
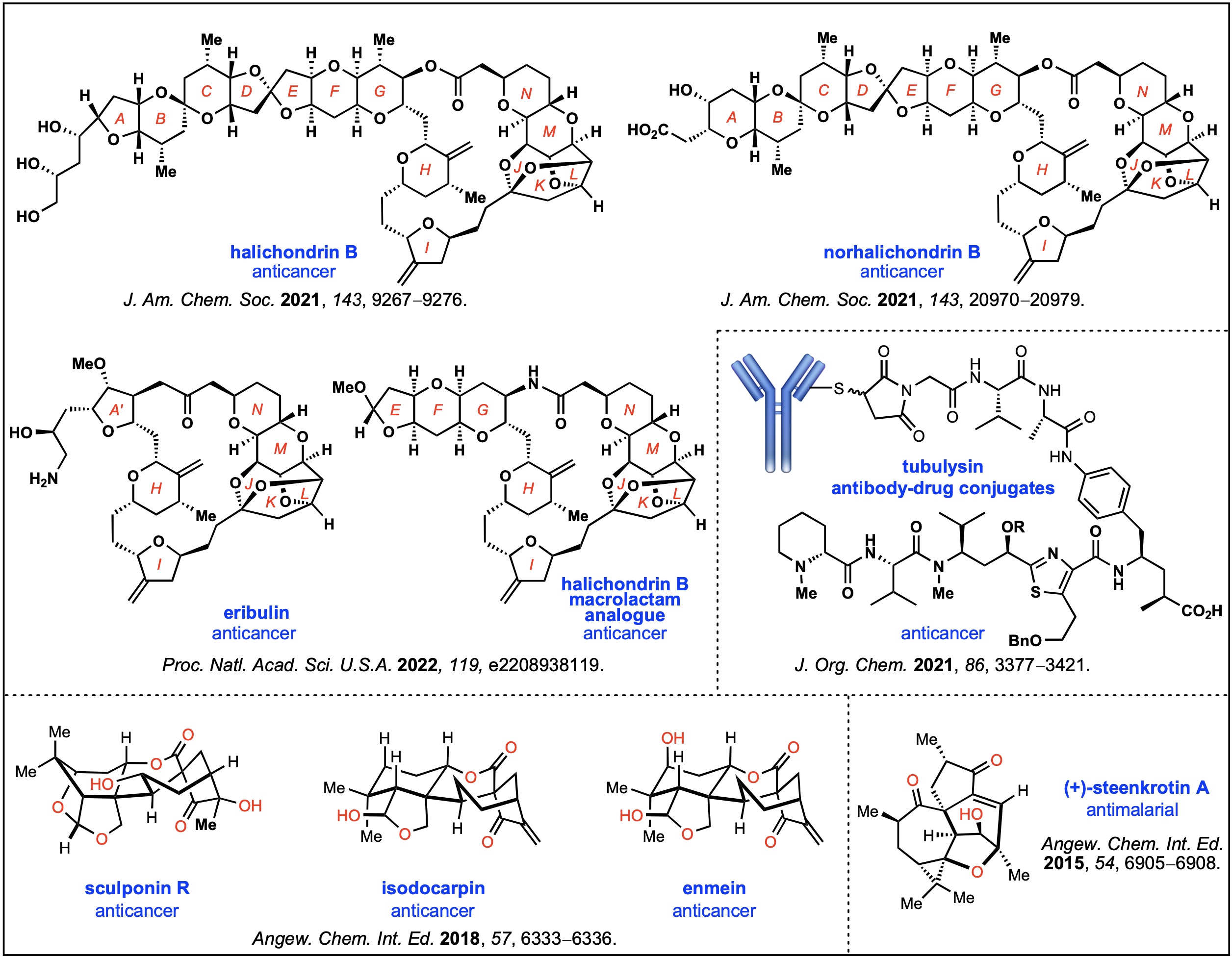
据最新调查,在过去40年批准的创新药中,有大约30%以上的新药来源于天然产物或者类似物。而在小分子创新药领域,这个比例高达40%以上。在抗体偶联药物领域,目前批准的或正在研究中的抗体偶联药物,其毒性药物基本上是来源于天然产物或类似物。因此,天然产物的全合成及其相关生物活性的研究,用于创新药物开发具有相当重要的科学和现实意义。 潘赛勇博士主要从事[活性天然产物全合成]研究工作,在围绕活性天然产物和复杂药物分子的高效全合成问题,开展并完成了一系列研究工作。通过多样性、汇聚式、模块化合成策略,创新、高效地完成了若干具有重要生物活性的复杂天然产物(halichondrin家族、enmein家族和(+)-steenkrotin A)和抗癌药物艾日布林(eribulin)的全合成,系统研究了抗癌天然产物tubulysin的构效关系和其抗体偶联药物的应用。在天然产物全合成及其药物化学领域取得了一系列成果,并得到了国内外同行的广泛关注和积极评价。
|
10. Nicolaou, K. C.*; Pan, S.; Shelke, Y.; Rigol, S.; Bao, R.; Das, D.; Ye, Q. A Unified Strategy for the Total Syntheses of Eribulin and a Macrolactam Analogue of Halichondrin B. Proc. Natl. Acad. Sci. U.S.A.2022, 119, e2208938119.
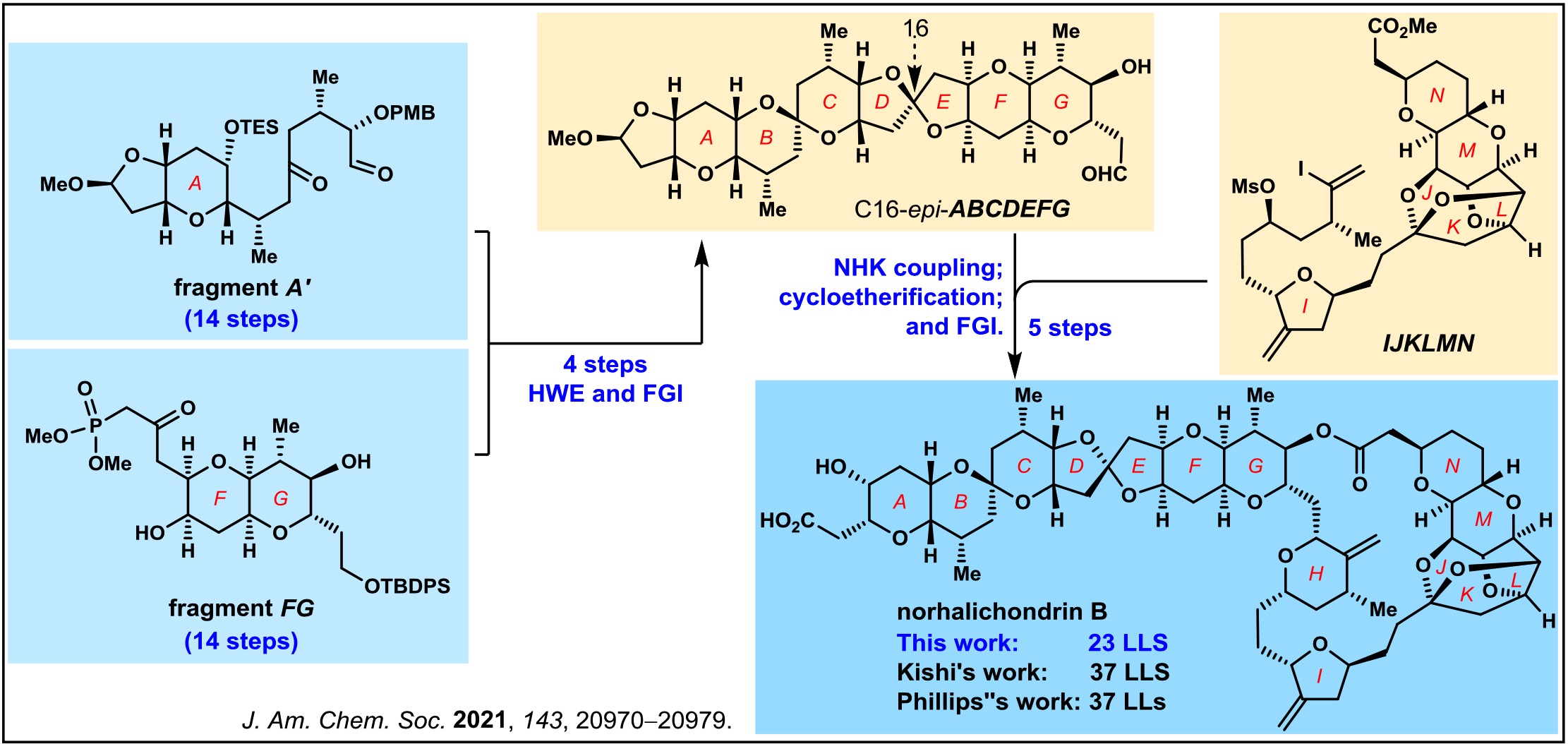
9. Nicolaou, K. C.*; Pan, S.; Shelke, Y.; Ye, Q.; Das, D.; Rigol, S., A Highly Convergent Total Synthesis of Norhalichondrin B. J. Am. Chem. Soc.2021, 143, 20970–20979.
8. Nicolaou, K. C.*; Pan, S.; Shelke, Y.; Das, D.; Ye, Q.; Lu, Y.; Sau, S.; Bao, R.; Rigol, S., A Reverse Approach to the Total Synthesis of Halichondrin B. J. Am. Chem. Soc. 2021, 143, 9267–9276.
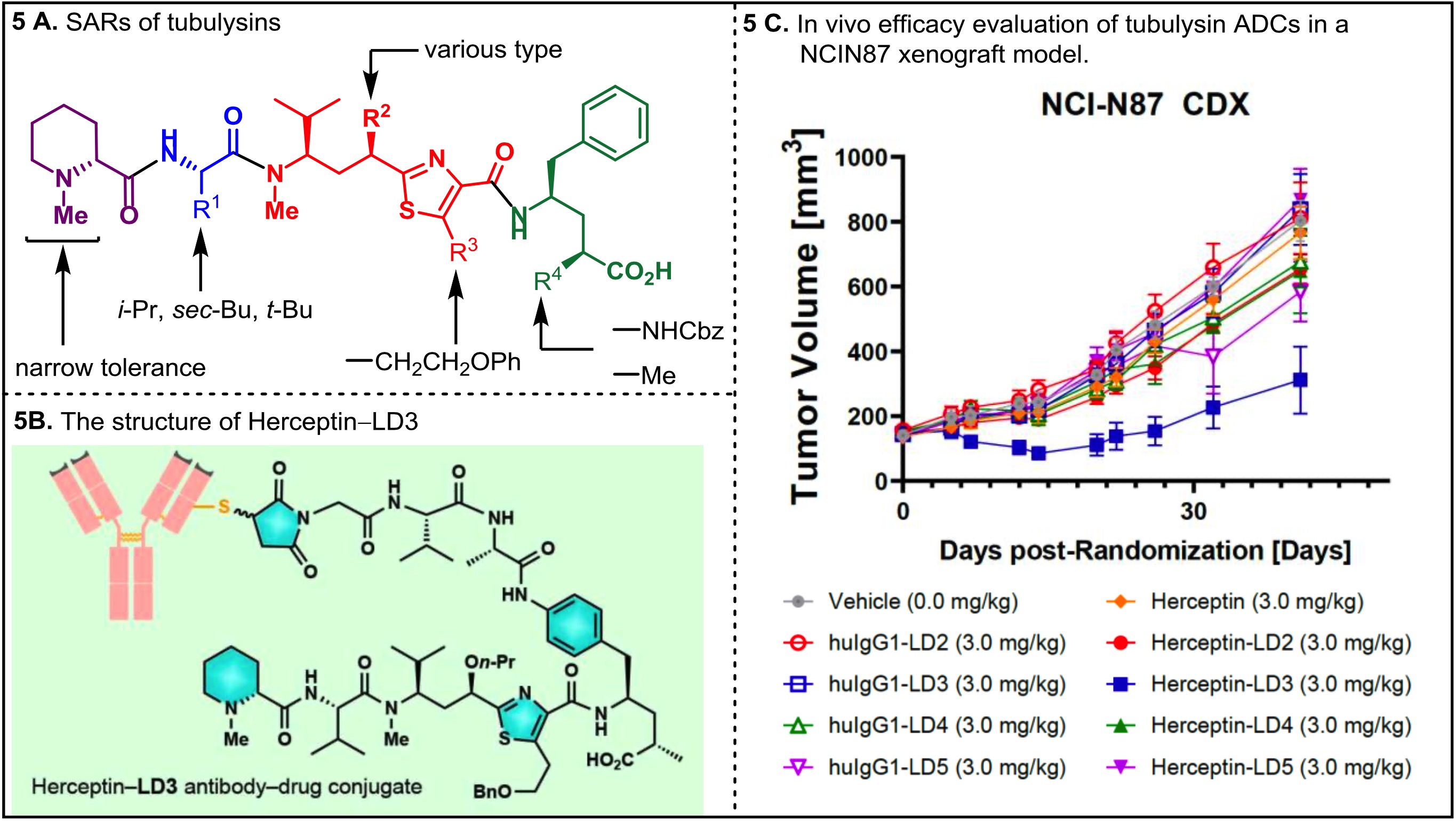
7. Nicolaou, K. C.*; Pan, S.; Pulukuri, K. K.; Ye, Q.; Rigol, S.; Erande, R. D.; Vourloumis, D.; Nocek, B. P.; Munneke, S.; Lyssikatos, J.; Valdiosera, A.; Gu, C.; Lin, B.; Sarvaiaya, H.; Trinidad, J.; Sandoval, J.; Lee, C.; Hammond, M.; Aujay, M.; Taylor, N.; Pysz, M.; Purcell, J. W.; Gavrilyuk, J., Design, Synthesis, and Biological Evaluation of Tubulysin Analogues, Linker-Drugs, and Antibody–Drug Conjugates, Insights into Structure–Activity Relationships, and Tubulysin–Tubulin Binding Derived from X-ray Crystallographic Analysis. J. Org. Chem. 2021,86, 3377–3421. (Pan, S.; Pulukuri, K. K. and Ye, Q contribute equally)
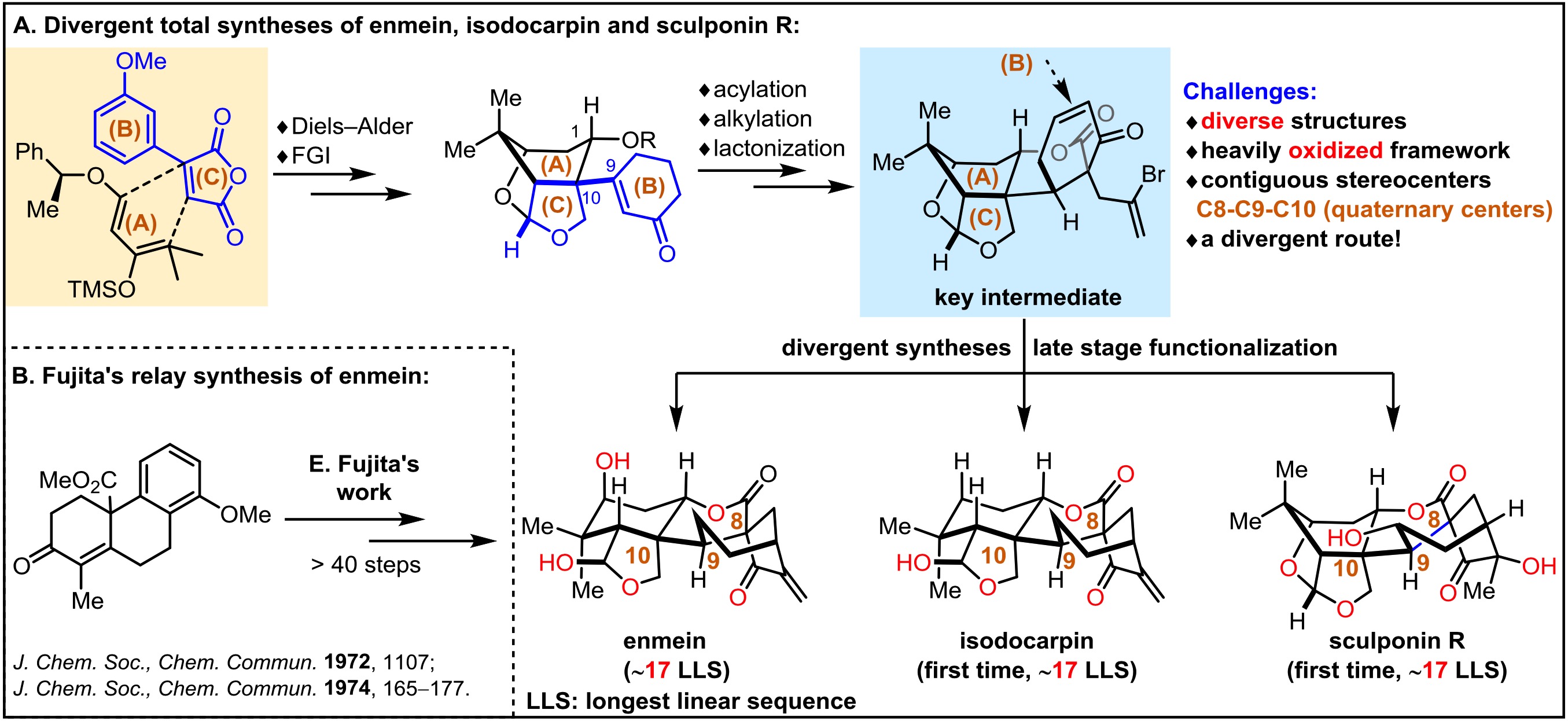
6. Pan, S.; Chen, S.; Dong, G*., Divergent Asymmetric Syntheses of Enmein-type Natural Products: (–)-Enmein, (–)-Isodocarpin, and (–)-Sculponin R. Angew. Chem. Int. Ed.2018,57, 6333–6336.
5. Pan, S.; Gao, B.; Hu, J.; Xuan, J.; Xie, H.; Ding, H.*, Enantioselective Total Synthesis of (+)-Steenkrotin A and Determination of Its Absolute Configuration. Chem. Eur. J.2016,22, 959–970. (Pan, S. and Gao, B. contribute equally) 4. Pan, S.; Xuan, J.; Gao, B..; Zhu, A.; Ding, H.*, Total Synthesis of Diterpenoid Steenkrotin A. Angew. Chem. Int. Ed. 2015,54, 6905–6908. (Pan, S. and Xuan, J. contribute equally)
3. Xuan, J.; Pan, S.; Zhang, Y.; Ye, B.; Ding, H.*, Construction of the Tricyclic Core of Steenkrotin-type Diterpenoids via Intramolecular [3+2] Cycloaddition. Org. Biomol. Chem. 2015,13, 1643–1646. (Xuan, J. and Pan, S. contribute equally) 2. Pan, S.; Zheng, L.; Nie, R.; Xia, S.; Chen, P.; Hou, Z.*, Transesterification of Glycerol with Dimethyl Carbonate to Glycerol Carbonate over Na-Based Zeolites. Chin. J. Catal. 2012,33, 1772–1777. 1. Nie, R.; Lei, H.; Pan, S.; Wang, L.; Fei, J.; Hou, Z.*, Core-shell Structured CuO-ZnO@H-ZSM-5 Catalysts for CO Hydrogenation to Dimethyl Ether. Fuel 2012,96, 419–425. |