
叶柏华课题组介绍
| 导师介绍 |
 叶柏华 Baihua Ye 助理教授、研究员、博导 (Assistant Professor) ❏ 通讯地址:上海科技大学浦东新区华夏中路393号,物质学院3号楼410-1 ❏ 电子邮件:yebh@@shanghaitech.edu.cn ❏ ORCID:orcid.org/0000-0001-9369-2656 ❏ 科研背景: 2018年9月-至今,上海科技大学物质科学与技术学院,担任助理教授、研究员、博导 2015年8月-2018年5月,美国加州大学伯克利分校 (UC Berkeley),博士后 (合作导师 F. Dean Toste 教授) 2010年10月-2015年6月,瑞士洛桑联邦理工学院 (EPFL),博士 (导师 Nicolai Cramer 教授) 2005年9月-2010年1月,瑞士洛桑联邦理工学院 (EPFL),学士 (导师 Jérôme Waser 教授) ❏ 课题组研究方向: 过渡金属催化,药物化学,多肽化学,聚合物化学,理论计算化学 ❏ 获奖荣誉: 国家海外高层次青年人才项目获得者(2020) 上海青年东方学者(2019) Swiss National Science Foundation Early Postdoc Mobility Fellowship (2015) Doctoral Thesis Award in Chemistry and Chemical Engineering (2015) Reaxys PhD Prize – Finalist (2014) Chinese Government Award for Outstanding Self-Financed Students Abroad (2013) ❏ 课题组网页:https://the-ye-group.com/ |
| 研究方向 |
课题组研究方向包括新型药物设计与合成、大宗化学品转化、有机催化工业化、聚合物合成与降解。具体为通过研发新型化学键官能团化为核心,运用各种有机催化与不对称合成策略,借此建立高效催化体系,进而提供解决生命健康相关挑战与绿色能源转化的新思路。
其中研究内容包括:
✅ 亲电交叉偶联(XEC)合成药物 ✅ 过渡金属有机协同催化
✅ 新型手性配体设计与合成制备 ✅ 过渡金属催化相关DFT理论计算
✅ 多肽分子修饰 ✅ 能源物质绿色转化 ✅ 功能聚合物设计与制备
| 研究工作 |
more interesting research coming soon!!!
[18] X. Li‡, F.-J. Liu‡, B. Ye* J. Org. Chem. 2025, accepted. ‡contributed equally “Asymmetric synthesis of tetrahydrofurans with vicinal stereocenters by RhII/RuII relay catalysis”
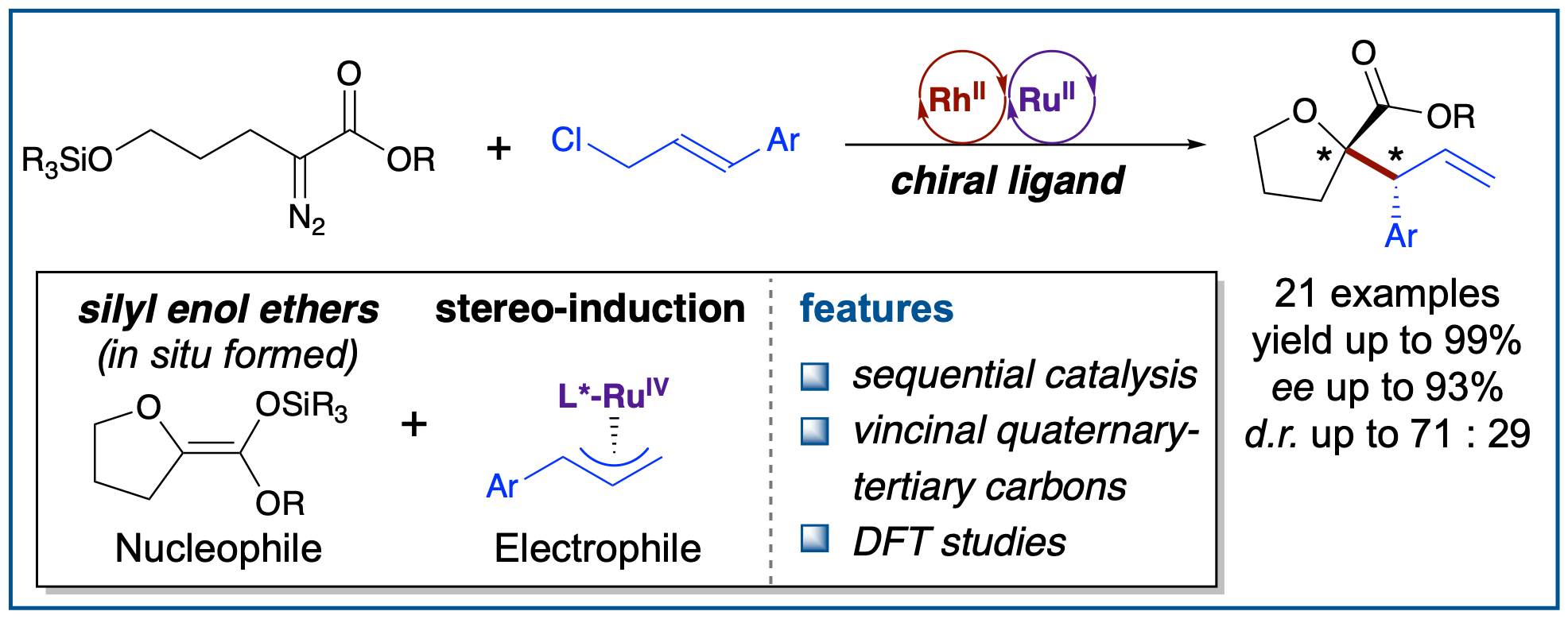
[17] Y. Gan§, X. Li§, J.-F. Zhou§, Baihua Ye* SYNLETT, 2025, accepted; §contributed equally (invited Synpacts); (doi: 10.1055/a-2589-5014) “Selective open-shell Ni catalysis driven by redox-transmetalation”
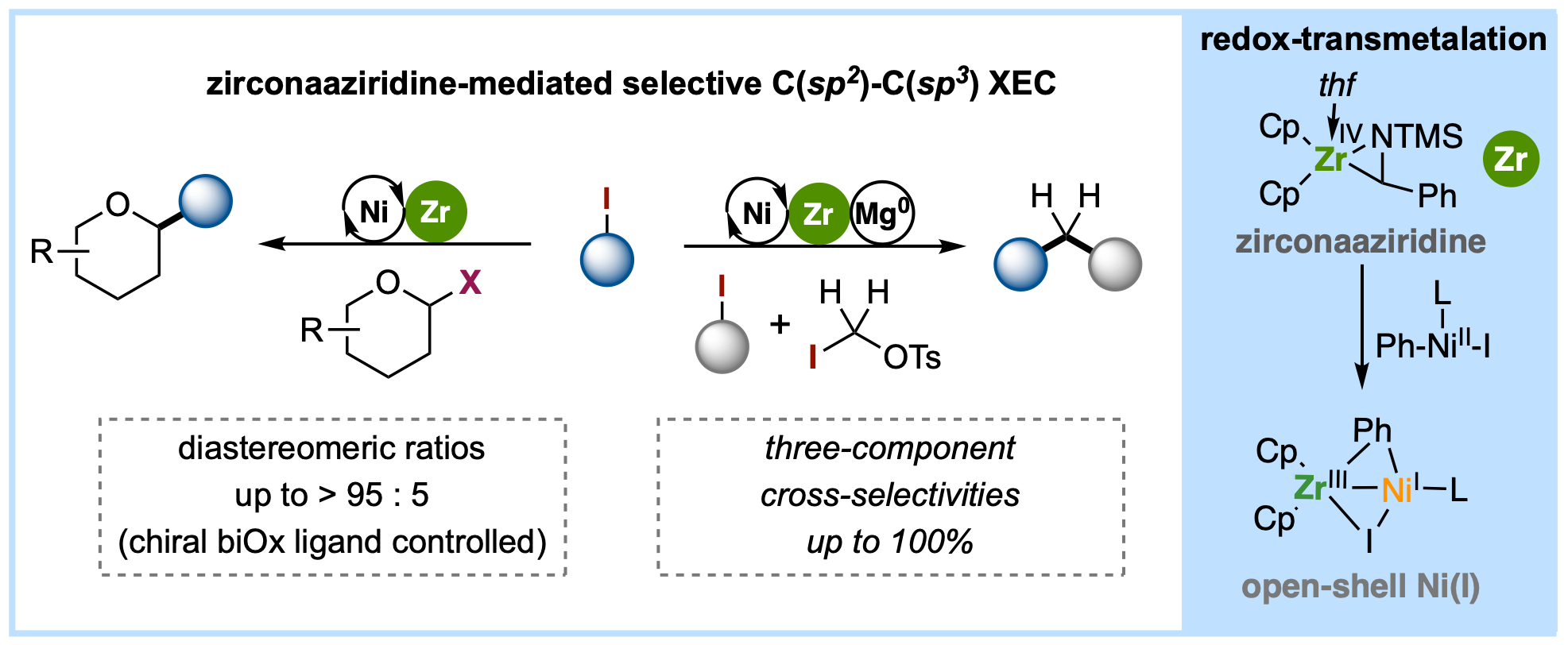
[16] Y. Gan§, J.-T. Tang§, X. Li, X.-S. Nie, Baihua Ye* ACS Sustain. Chem. Eng. 2025, 13, 3089-3096. §contributed equally “Valorization of Polyester Plastics and Biomass into Amines Through a Dual Zirconium Catalysis” (doi: 10.1021/acssuschemeng.4c08239)
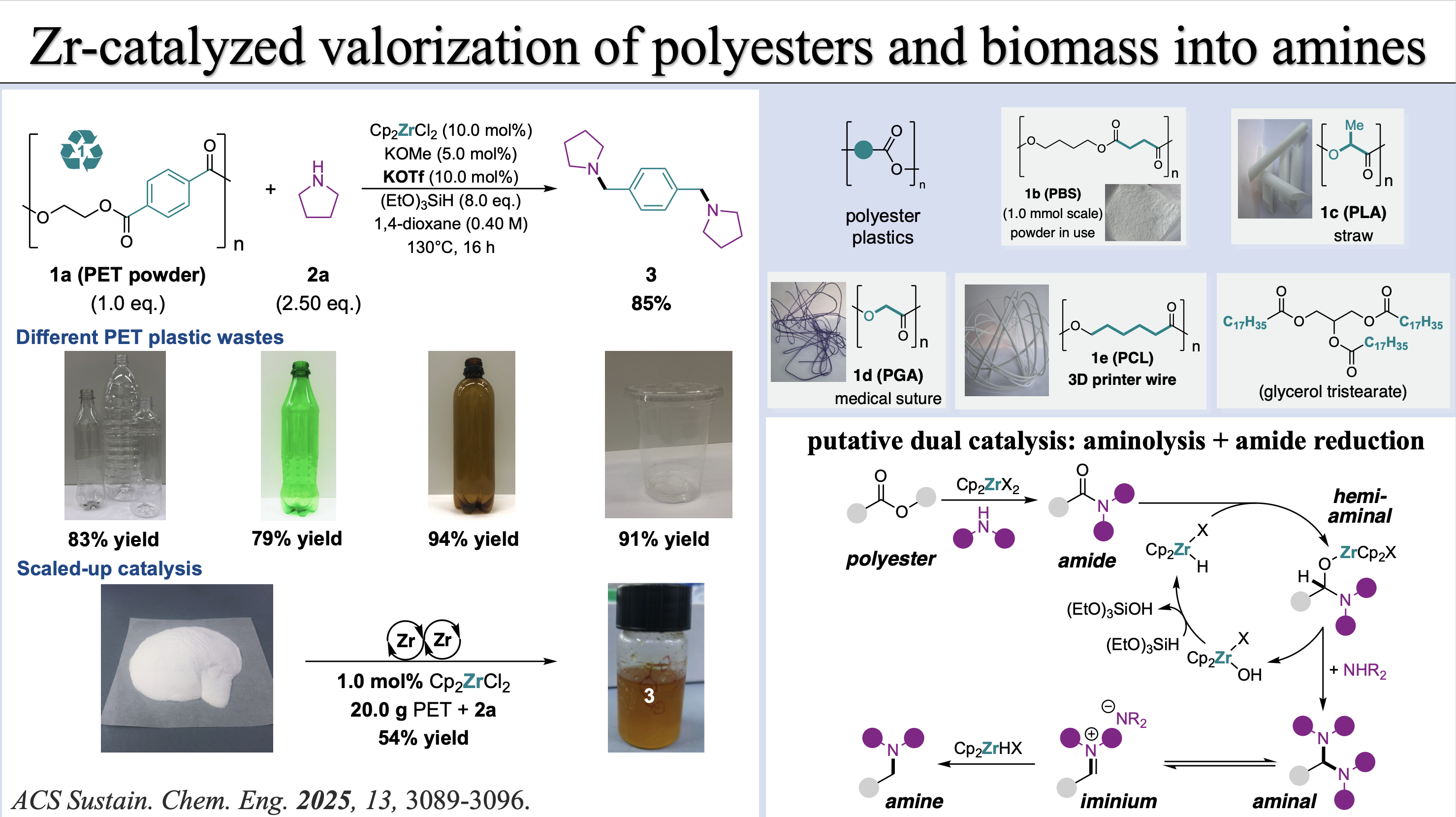
[15] X. Li, Y. Gan, Y.-Y. Wang, Baihua Ye* J. Am. Chem. Soc. 2024, 146, 35275–35284. “Selective Ni(I)/Ni(III) Process for Consecutive Geminal C(sp3)-C(sp2) Bond Formation” (doi: 10.1021/jacs.4c12581)
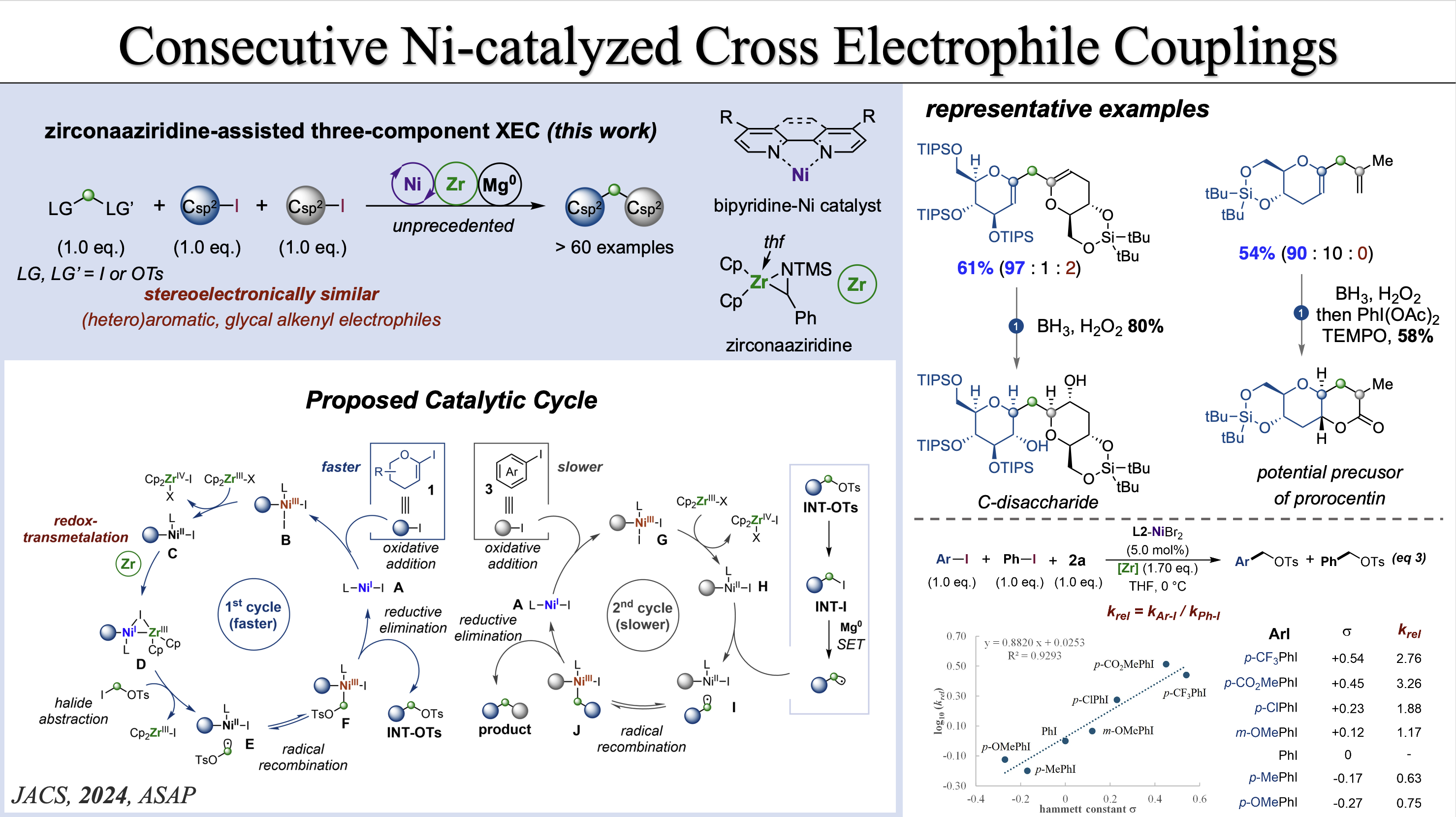
[14] Y. Gan§, J.-F. Zhou§, X. Li, J.-R. Liu, F.-J. Liu, X. Hong*, Baihua Ye* J. Am. Chem. Soc. 2024, 146, 16753-16763. §contributed equally “Zirconaaziridine-Mediated Ni-Catalyzed Diastereoselective C(sp2)‑Glycosylation”. (doi: 10.1021/jacs.4c04587)

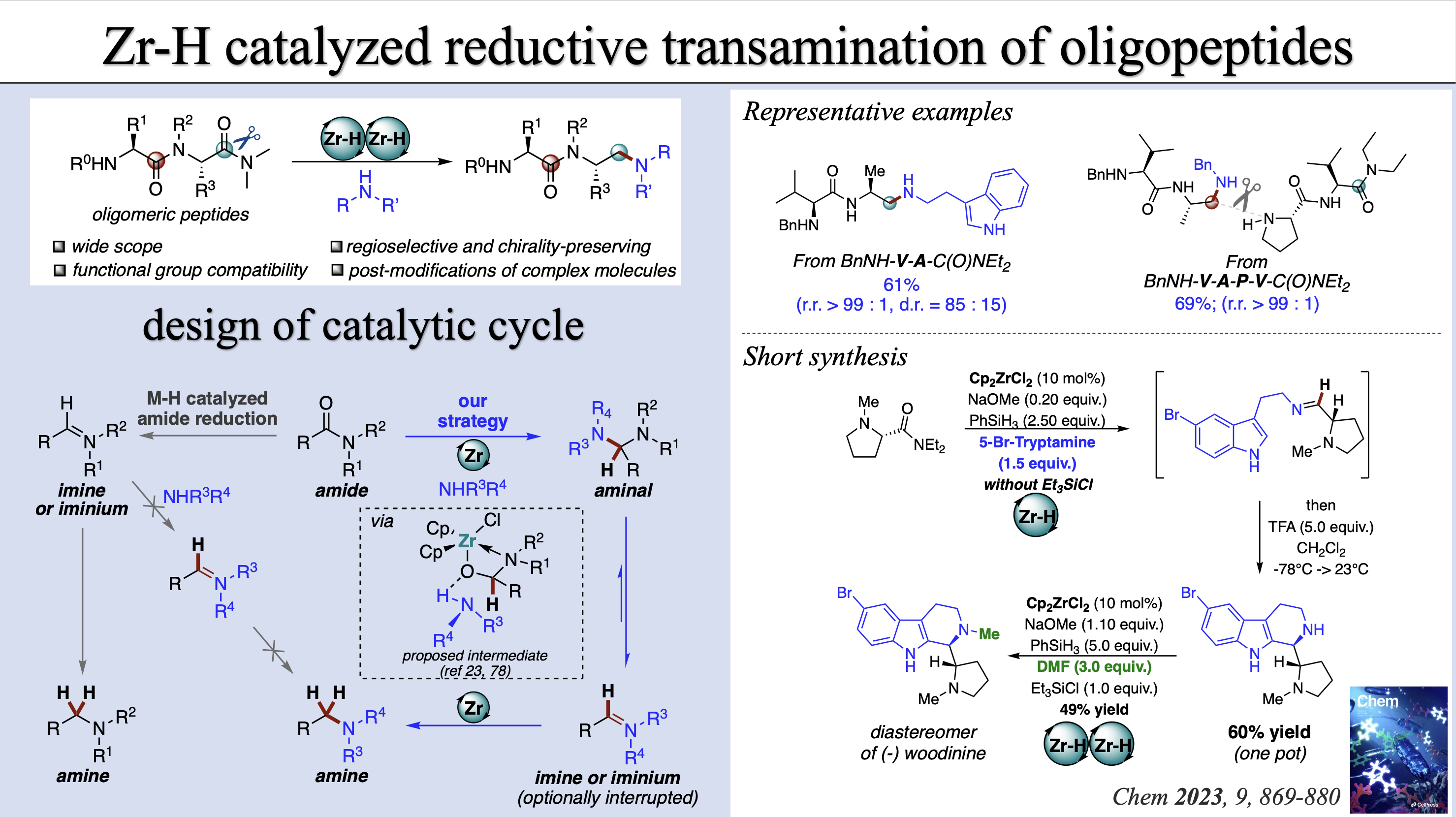
[13] J.-T. Tang§, Y. Gan§, X. Li§, Baihua Ye* Chem 2023, 9, 869-880. §contributed equally “Regioselective reductive transamination of peptidic amides enabled by a dual Zr(IV)–H catalysis”. (doi: 10.1016/j.chempr.2022.11.002)
[12] book chapter entitled Hydrometallation of Organometallic Complexes; J. Zhao*, Baihua Ye* Comprehensive Organometallic Chemistry IV, 2022 (章节内容:过渡金属催化)

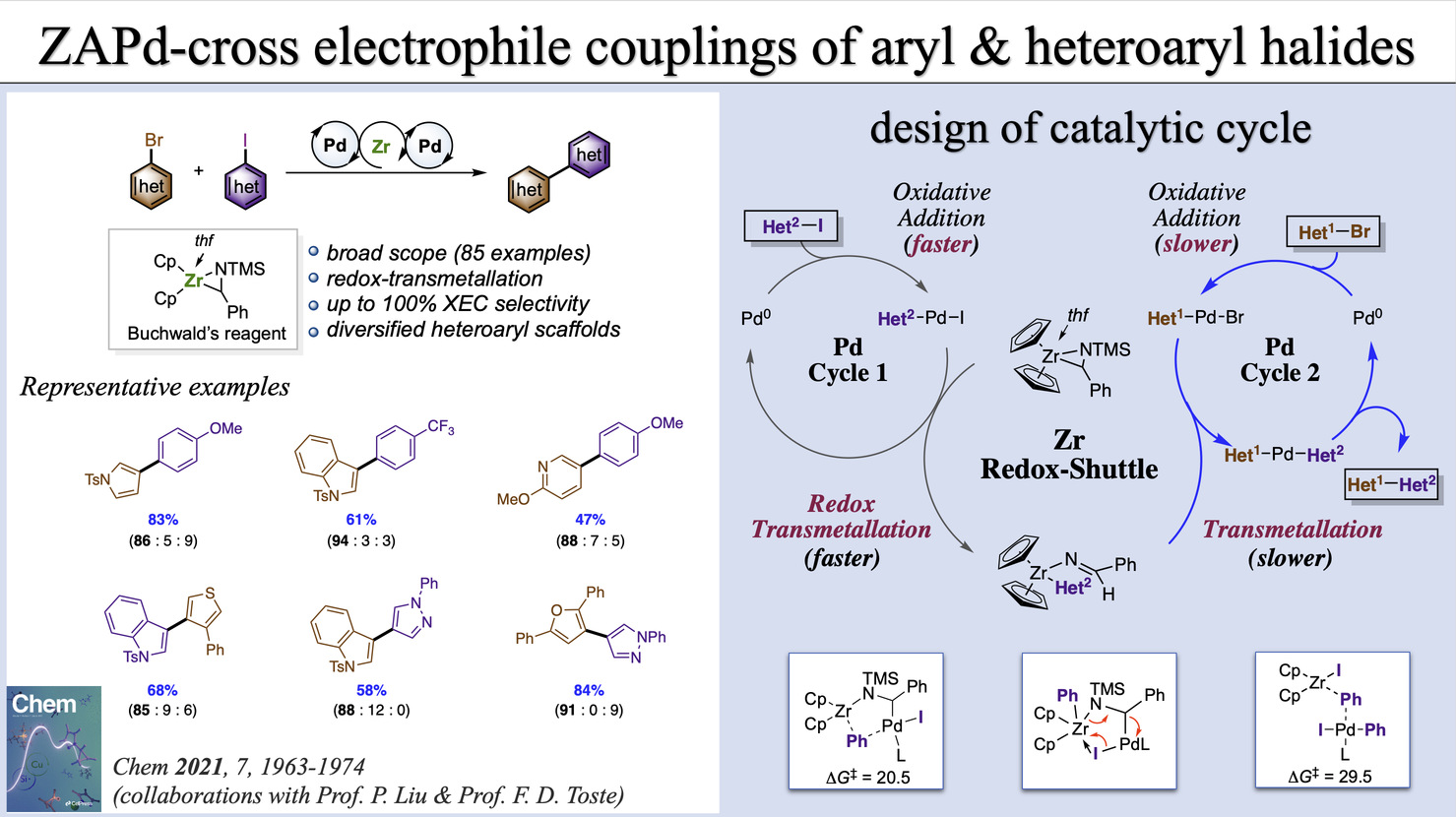
[11] T.-F. Wu§, Y.-J. Zhang§, Y. Fu§, F.-J. Liu, J.-T. Tang, P. Liu,* F. D. Toste,* Baihua Ye* Chem 2021, 7, 1963-1974. §contributed equally “Zirconium-redox-shuttled cross-electrophile coupling of aromatic and heteroaromatic halides” (doi: 10.1016/j.chempr.2021.06.007)
Chem & Chem Catalysis编辑精选 (https://mp.weixin.qq.com/s/xQCwR0eDq81seo_SqJjtqw)
ShanghaiTech公众号报道(https://www.shanghaitech.edu.cn/2021/0714/c1001a67866/page.htm)
Ph.D & Postdoc Research:
[10] Baihua Ye§, Jie Zhao§, Ke Zhao, Jeffrey M. McKenna, F. Dean Toste* J. Am. Chem. Soc. 2018, 140, 8350-8356. §contributed equally
“Chiral Diaryliodonium Phosphate Enables Light Driven Diastereoselective a-C(sp3)-H Acetalization”
[9] Jie Zhao§, Son C. Nguyen§, Rong Ye§, Baihua Ye§, Horst Weller, Gabor A. Somorjai*, A. Paul Alivisatos*, F. Dean Toste* ACS Cent. Sci. 2017, 3, 482-488. §contributed equally
'A Comparison of Photocatalytic Activities of Gold Nanoparticles Following Plasmonic and Interband Excitation and a Strategy for Harnessing Interband Hot Carriers for Solution Phase Photocatalysis'
[8] Baihua Ye, Nicolai Cramer* Acc. Chem. Res. 2015, 48, 1308-1318.
“Chiral Cyclopentadienyls: Enabling Ligands for Asymmetric Rh(III)-Catalyzed C-H Functionalizations”
[7] Baihua Ye, Nicolai Cramer* Synlett 2015, 26, 1490-1495.
“Chiral Cyclopentadienyl Ligands Enable a Rhodium(III)-Catalyzed Enantioselective Access to Hydroxychromames and Phthalides”
[6] Matthew D. Wodrich§, Baihua Ye§, Jérôme. F. Gonthier, Clémence Corminboeuf, Nicolai Cramer* Chem. Eur. J. 2014, 20, 15409-15418. §contributed equally
“Ligand-Controlled Regiodivergent Pathways of Rhodium(III)-Catalyzed Dihydroisoquinolone Synthesis: Experimental and Computational Studies of Different Cyclopentadienyl Ligands”
[5] Baihua Ye, Nicolai Cramer* Angew. Chem. Int. Ed. 2014, 53, 7896-7899.
“Asymmetric Synthesis of Isoindolones by Chiral Cyclopentadienyl-Rhodium(III)-Catalyzed C-H Functionalizations”
[4] Baihua Ye, Pavel A. Donets, Nicolai Cramer* Angew. Chem. Int. Ed. 2014, 53, 507-511.
“Chiral Cp-Rhodium(III)-Catalyzed Asymmetric Hydroarylations of 1,1-Disubstituted Alkenesˮ
[3]Baihua Ye, Nicolai Cramer* J. Am. Chem. Soc. 2013, 135, 636-639.
“A Tunable Class of Chiral Cp Ligands for Enantioselective Rhodium(III)-Catalyzed C-H Allylations of Benzamidesˮ
[2]Baihua Ye, Nicolai Cramer* Science 2012, 338, 504-506.
“Chiral Cyclopentadienyl Ligands as Stereocontrolling Element in Asymmetric C-H Functionalizationˮ
[1] Manh V. Pham, Baihua Ye, Nicolai Cramer, Angew. Chem. Int. Ed. 2012, 51, 10610-10614.
“Access to Sultams by Rhodium(III)-Catalyzed Directed C-H Activationsˮ
| 课题组 |