Liquid sorbents, such as water, polyethylene glycol (PEG), and organic amine solutions, are widely used in many industrial processes for acid gases removal. This process is called wet scrubbing. However, most liquid sorbents have high vapor pressure and are sometimes even corrosive to the equipment. These undesirable features greatly increase the cost of separation and complicate facility design. In contrast, solid adsorbents are non-corrosive and have no vapor pressure. They exhibit sorption behaviors far richer than their liquid counterparts due to the presence of permanent pores. One representative class of porous materials is metal organic frameworks (MOFs). However, conventional knowledge tells us that permanent porosity only exists in solid materials, meaning solid sorbents cannot flow and liquid sorbent materials cannot have permanent porosity. Recently, Li Tao 's research group used a new surface modification technique to achieve stable MOF particle suspension in a bulk polymeric solvent, polydimethylsiloxane (PDMS). The resultant materials, namely porous liquids (PLs), flow like liquids but at the same time, possess all the permanent porosity from the MOFs. These PLs combines the merits of both solid and liquid sorbents, which offer new insights for industrial gas separation. The work, “General Way To Construct Micro- and Mesoporous Metal–Organic Framework-Based Porous Liquids”, was published in Journal of the American Chemical Society.
In this work, researchers use surface-functionalized MOF particles as porous fillers and liquid PDMS as bulky solvents. Using a generalizable non-covalent surface-initiated controlled radical polymerization technique, a series of isoreticular UiO-66 particles were dispersed in a liquid PDMS solvent with excellent homogeneity and colloidal stability. Benefiting from the inherent properties of PDMS, the PLs exhibit low vapor pressure, high thermal stability, and fluidity down to -35°C. Attributing to the bulkiness of PDMS and its inherent high permeability, the sorption properties of the MOF fillers can be largely retained in their respective PLs as confirmed by low pressure CO2, Xe and H2O sorption isotherms. The permanent porosity of the PLs can also be largely preserved even after 15 months of storage. Moreover, they demonstrated that by tuning the molecular weight and polymer chain architecture of PDMS, it is possible to preserve the permanent porosity of a mesoporous MOF, MIL-101(Cr) (mesopores as large as 3.4 nm), within a PL.
All the research was done at ShanghaiTech University. Fifth year graduate student He Sanfeng from Li Tao’s research group is the lead author of the paper. Assistant Professors Li Tao and Yongjin Lee are corresponding authors. The molecular dynamics simulation (MD simulation) experiments in this research were conducted by Cui Jing, a graduate student from Yongjin Lee’s group. Biao Yuan from Yu Yi’s group assisted with the energy dispersive X-ray spectrum (EDS) measurement. This is a perfect example of cross-collaboration among SPST research groups.
Read more at: https://pubs.acs.org/doi/10.1021/jacs.9b08458
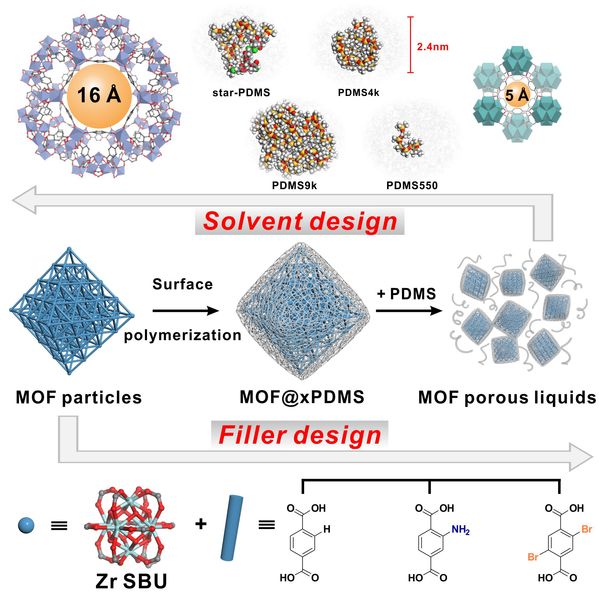
Figure 1. Schematic illustration of surface engineering, filler design and solvent design for the construction of MOF-based PLs.
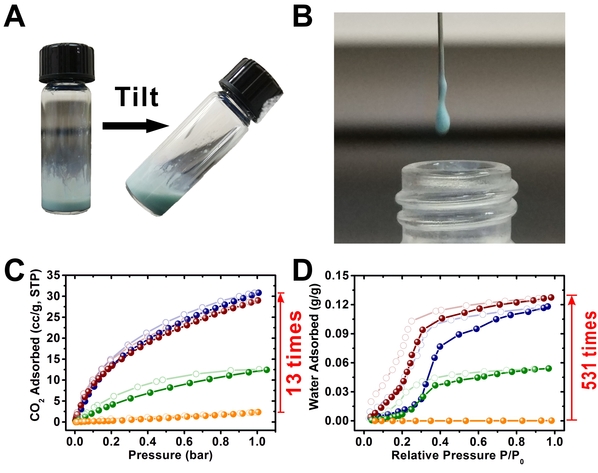
Figure 2. (A) (B) Photographs of PLs; (C) CO2 sorption isotherms at 273 K and (D) water vapor sorption isotherms at 298 K of PLs after 3 months storage. Compared to pure PDMS solvent, PL exhibits a 13-fold increase in CO2 uptake capacity and 531-fold increase in water uptake capacity.


