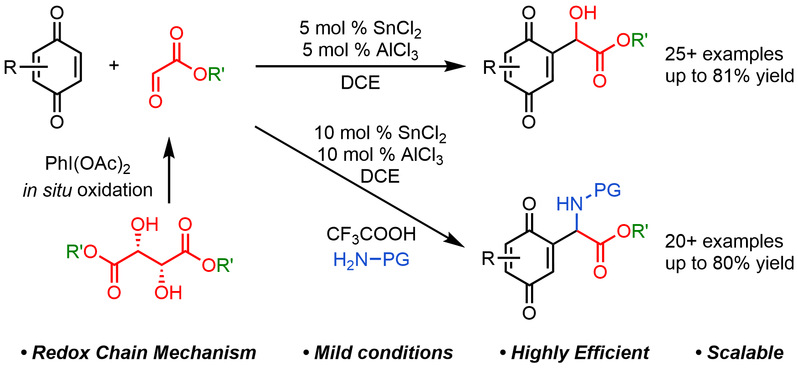
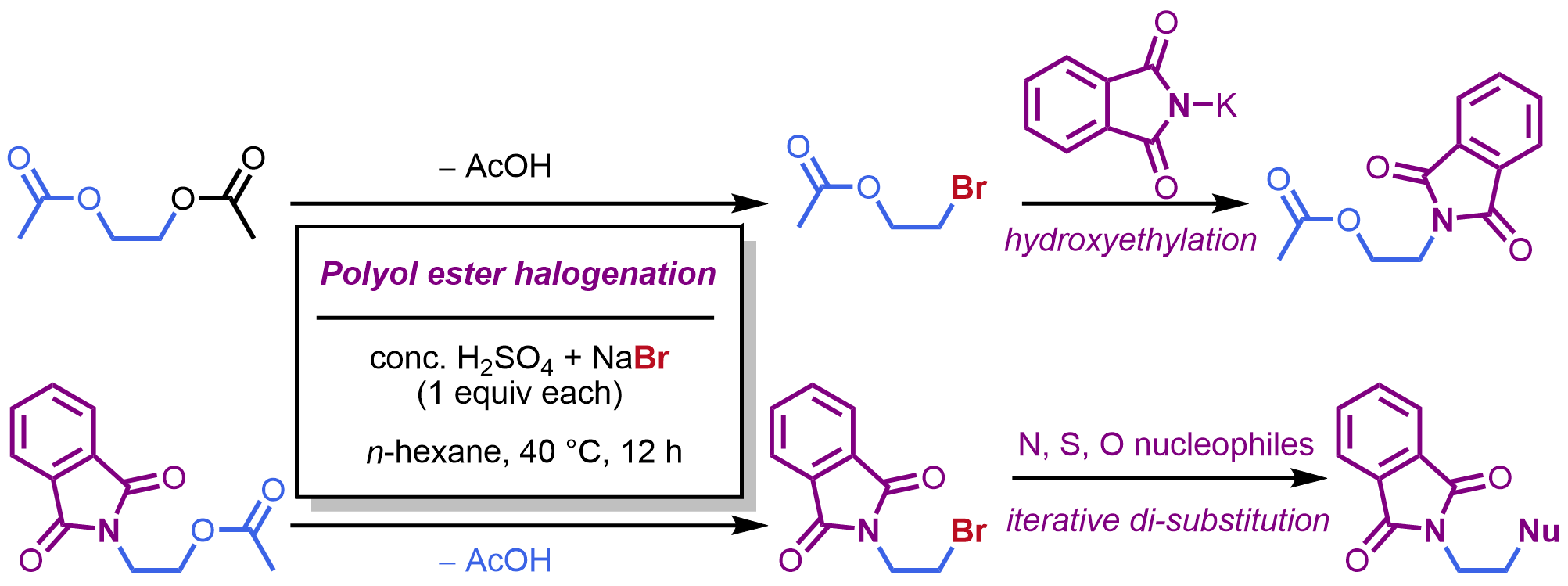
Independent Publications: Ye, Y.-M.; Li, Z.*; Construction of α-quinonyl-α-hydroxy/amino acid esters through the redox chain reaction, Chem. Sci., 2025, 16, 20564-20569. (Open access) Wan, Y.-D.; Li, Z.*; Easily accessible halohydrin esters as advantageous hydroxyethylation reagents, Synlett, 2025, 36, 1530-1534. Ye, Y.-M.; Chen, H.-W.; Gu, H.; Qiao, B.; Li, Z.*; A Flash Conversion to Aromatic Azo Compounds Expedited by Hydrazine–Trifluoroacetate Hydrogen Bonding, Org. Lett., 2025, 27, 4450-4456. 

See the video for how fast the reaction can go:
|
|
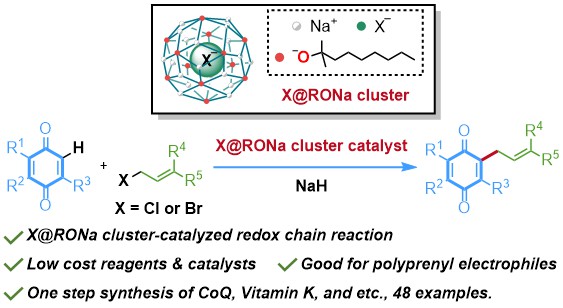
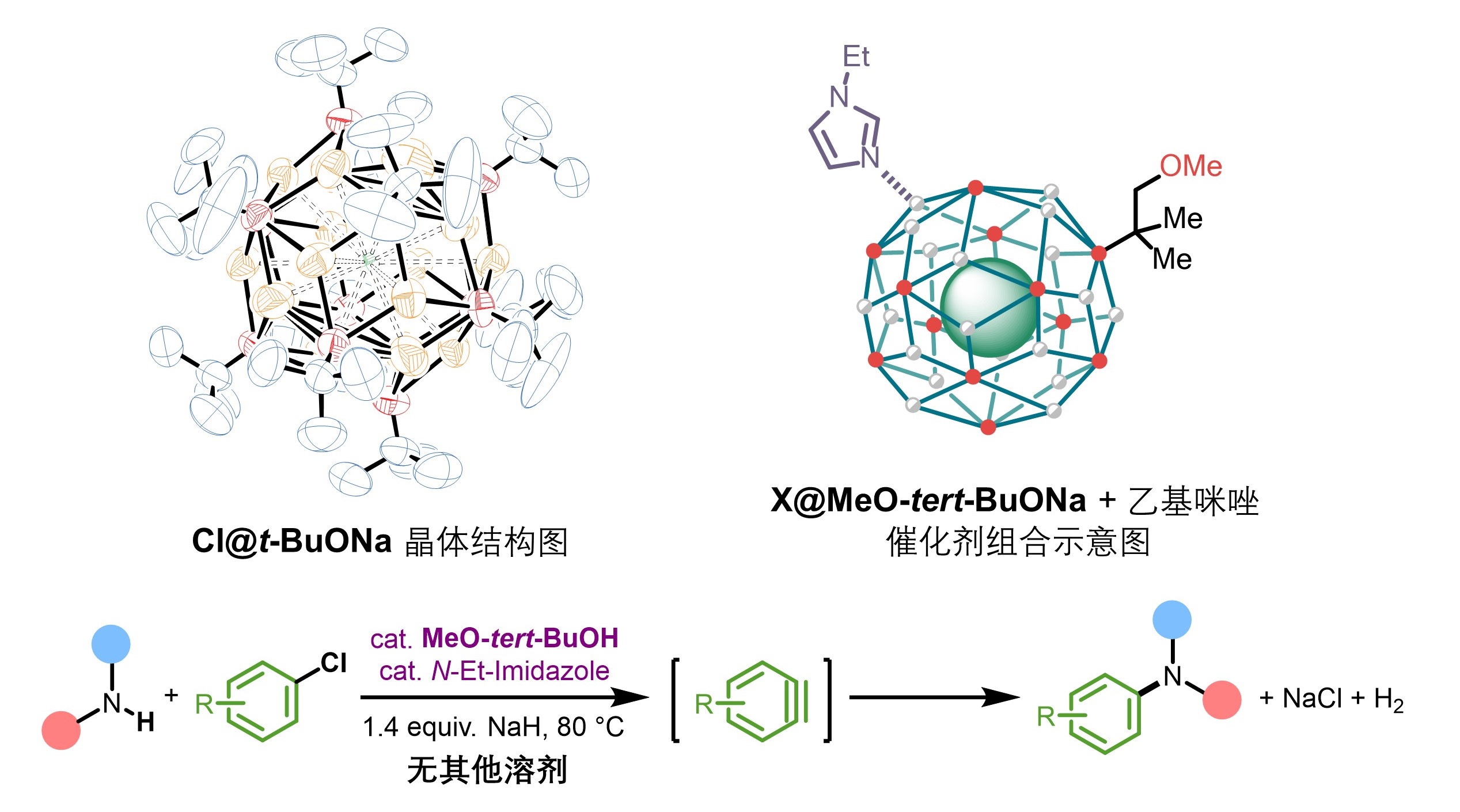
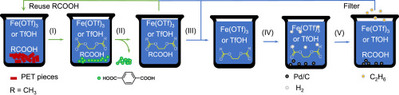
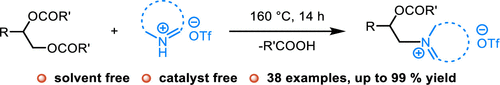
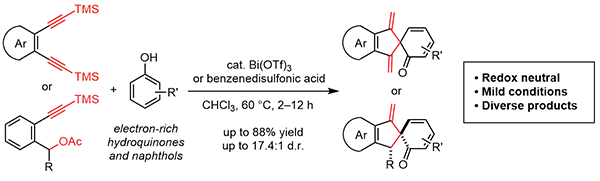
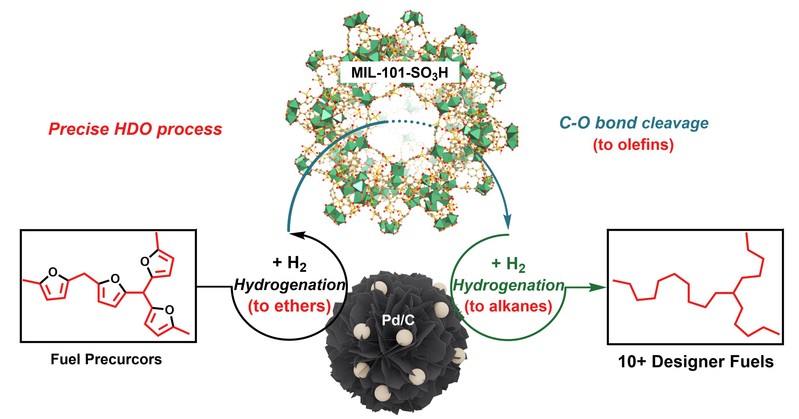
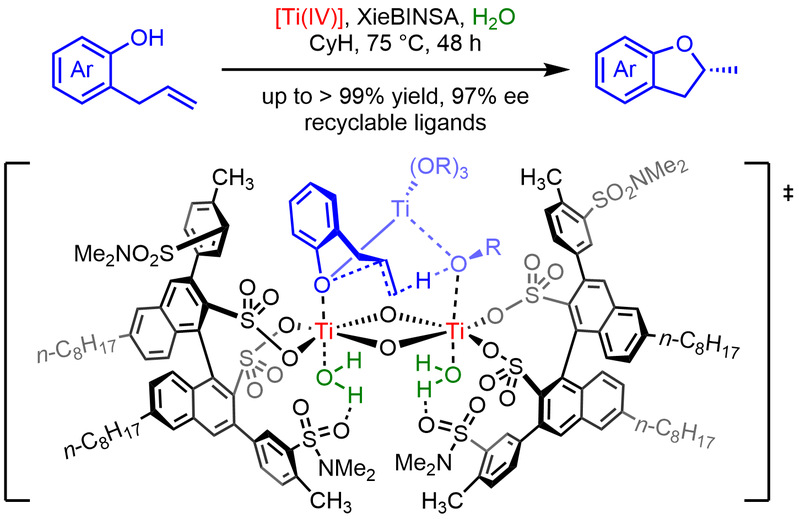
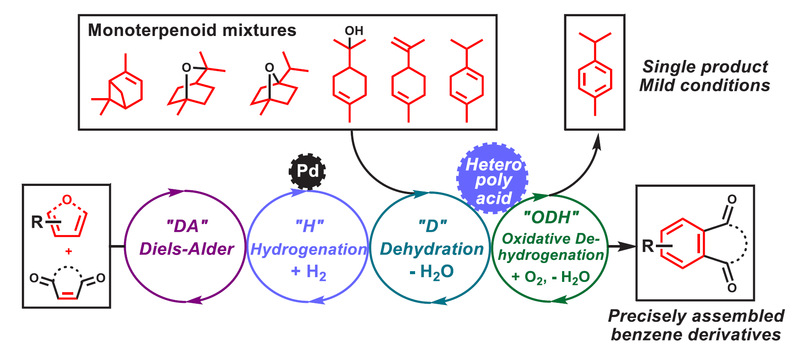
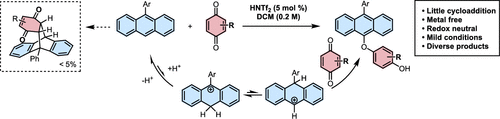
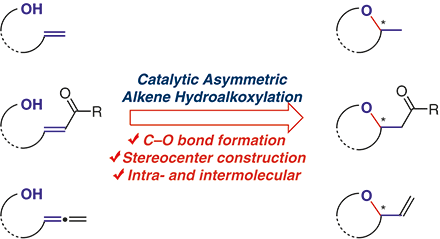
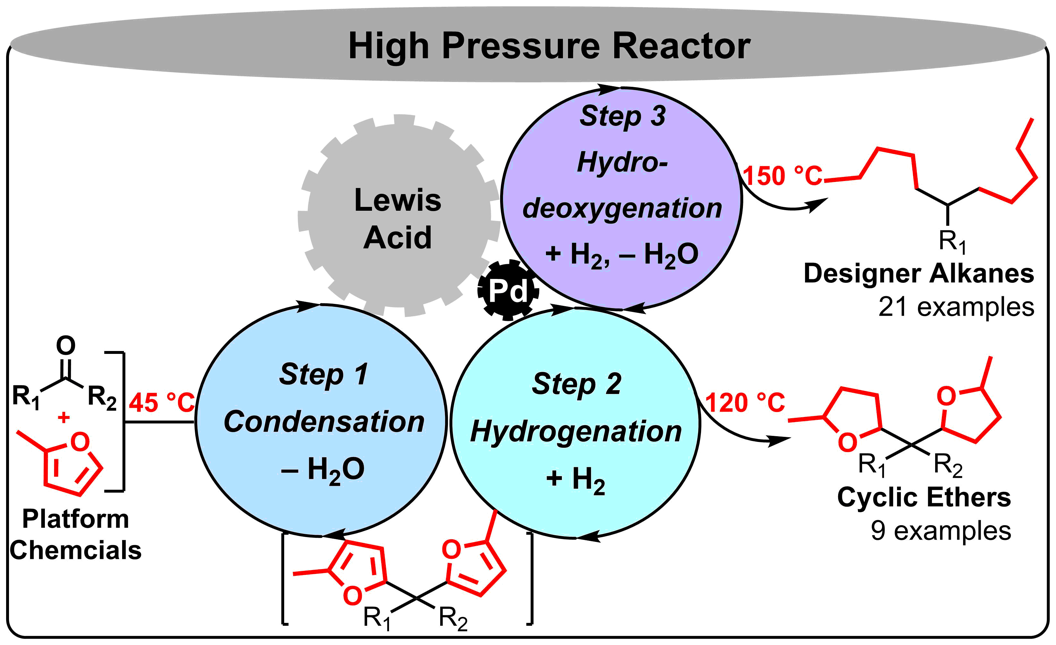
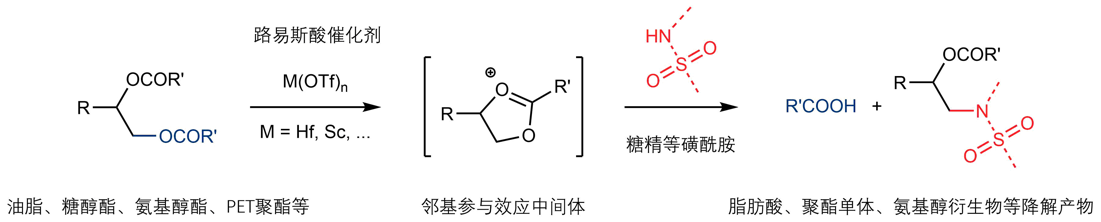
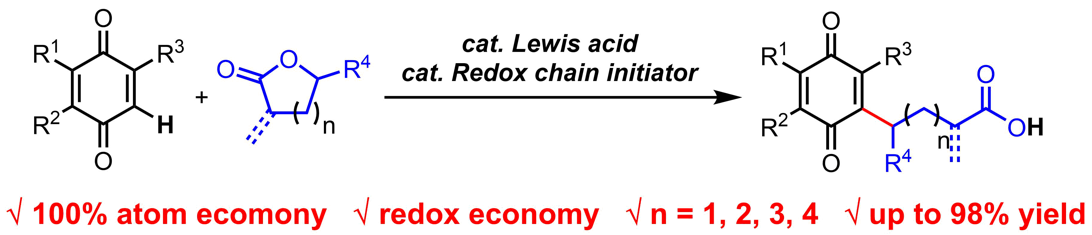
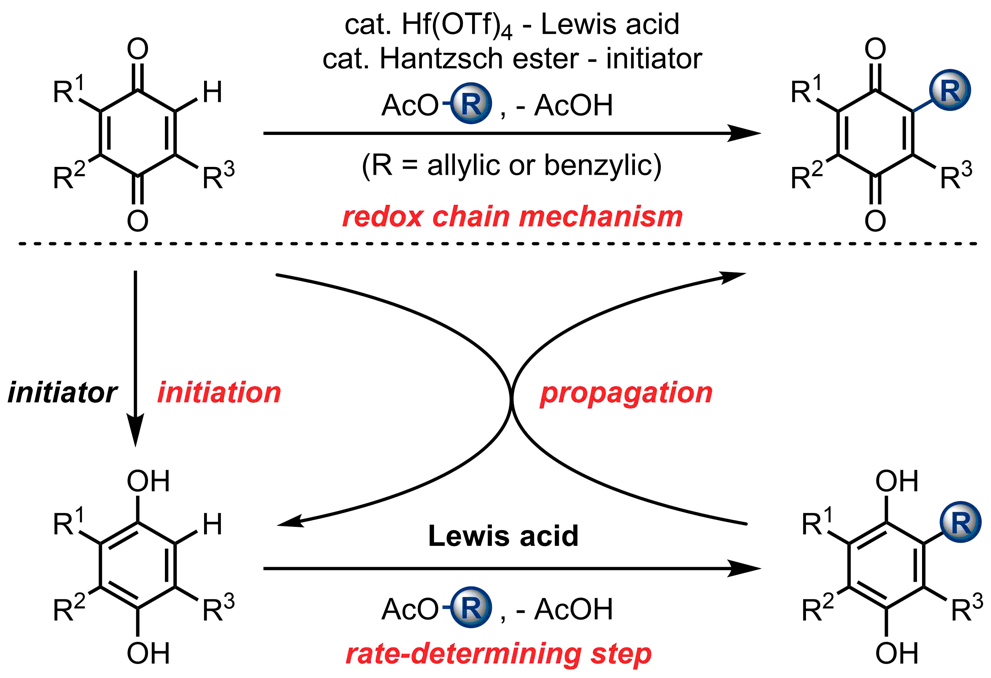
Tang, C.; Zhang, R.*; Almunif, S.; Das, P. J.; Brown, P. J.; Young, R. M.; Wu, G.; Han, H.; Zhao, X.; David, A. H. G.; Wu, H.; Song, B.; Abhervé, A.; Wu, Y.; Ye, Y.-M.; Feng, Y.; Chen, A. X.-Y.; Stern, C. L.; Li, Z.; Scott, E. A.; Wasielewski, M. R.; Stoddart, J. F.; A compact catenane with tuneable mechanical chirality, Nat. Synth. 2025, 4, 956-964. Cao, X.-L.; Yao, J.-L.; Shen Z.-N.; Li, Z.; Qiao, B.*; Utilizing Noncovalent Interactions to Lock and Facilitate Bond Rotation Enables Wide-Range Modulation of the Thermal Isomerization Dynamics of Photoswitches, CCS Chem. 2025, 7, 786-797. (Open access) Zhang, Z.; Gu, H.; Cao, D.-X.; Li, Z.*; Rapid Access to Isoprenoid Quinones through X@RONa-Catalyzed Redox Chain Reaction, J. Am. Chem. Soc. 2024, 146, 29064–29071. (Open access) Yao, J.-L.; Li, Z.*; Hafnium(IV)-Salen-Catalyzed Highly Reactive and Enantioselective Epoxidation Directed by Amides, ACS Catal. 2024, 14, 12494–12499. (Featured in the Organic Chemistry Portal: https://www.organic-chemistry.org/Highlights/2025/21July.shtm) Luo, Y.-J.; Li, Z.*; Catalytic Hydroxyethylation of Phenols with Renewable Ethylene Glycol Diester as an Alternative to Ethylene Oxide, Eur. J. Org. Chem. 2024, 27, e202400158. Ye, Y.-M.; Li, Z.*; Olefins Hydrofunctionnalization; Hydroamination and Hydroalkoxylation of Olefins, Excluding Electron Deficient Olefins. In Comprehensive Chirality, 2nd edition; Cossy, J., Ed., Elsevier: Academic Press, 2024; pp 119–150. Yao, J.-L.; Zhang, Z.; Li, Z.*; Scalable Transition-Metal-Free Synthesis of Aryl Amines from Aryl Chlorides through X@RONa-Catalyzed Benzyne Formation, J. Am. Chem. Soc. 2024, 146, 8839-8846. (Featured in Synfacts and selected as Synfact of the month: Synfacts 2024, 20(07), 0729. Featured in the Organic Chemistry Portal: https://www.organic-chemistry.org/abstracts/lit9/509.shtm) Luo, Y.-J.; Sun, J.-Y.; Li, Z.*; Rapid chemical recycling of waste polyester plastics catalyzed by recyclable catalyst, Green. Chem. Eng. 2024, 5, 257-265. (Open access) Wang, T.; Shi, S.; Shi, Y.; Jiang, P.; Hu, G.; Ye, Q.; Shi, Z.; Yu, K.; Wang, C.; Fan, G.; Zhao, S.; Ma, H.; Chang, A. C. Y.; Li, Z.; Bian, Q.*; Lin, C.-P.*; Chemical-induced phase transition and global conformational reorganization of chromatin, Nat. Commun. 2023, 14, 5556. (Open access) Shi, Z.; Bian, Q.*; Li, Z.*; Access to N-Alkylazaheterocyclic Salts by Activation of Alkoxy C–O Bonds in Polyol Esters, J. Org. Chem. 2023, 88, 9769–9782. Ding, N.; Li, Z.*; Acid-catalyzed [4+1] dearomatization spiroannulation of hydroquinones and naphthols, Synlett; 2023, 34, 2417-2422. (Special issue dedicated to Prof. H. Yamamoto) Liu, D.-H.; He, H.-L.; Wang, J.-J.; Zhou, S.-Y.; Zeng, T.; Gao, X.-Y.; Xiao, Y.; Yi, X.; Zheng, A.; Zhang, Y.-B.; Li, Z.*; Acidic metal–organic framework empowered precise hydrodeoxygenation of bio-based furan compounds and cyclic ethers for sustainable fuels, Green. Chem. 2021, 23, 9974-9981. Xie, W.-B.; Li, Z.*; Bis(μ-oxo)–Dititanium(IV)–Chiral Binaphthyldisulfonate Complexes for Highly Enantioselective Intramolecular Hydroalkoxylation of Nonactivated Alkenes, ACS Catal., 2021, 11, 6270-6275. Liu, X.; Liu, B.; Shi, Z.; Tan, C.; Fan, R.; Li, Z.; Tan, J.*; Hf(OTf)4-Catalyzed 1,6-Conjugate Addition of 2-Alkyl-azaarenes to para-Quinone Methides, J. Org. Chem. 2021, 86, 3615-3624. Liu, D.-H.; He, H.-L.; Zhang, Y.-B.; Li, Z.*; Oxidative Aromatization of Biobased Chemicals to Benzene Derivatives through Tandem Catalysis, ACS Sustainable Chem. Eng. 2020, 8, 14322–14329. Ding, N.; Li, Z.*; When Anthracene and Quinone Avoid Cycloaddition: Acid-Catalyzed Redox Neutral Functionalization of Anthracene to Aryl Ethers, Org. Lett. 2020, 22, 4276-4282. (CBG: https://www.chembeango.com/zixun/50298) Xie, W.-B.; Li, Z.*; Asymmetric Synthesis of Ethers by Catalytic Alkene Hydroalkoxylation, Synthesis 2020, 52, 2127-2146. (Invited review) Liu, D.-H., Marks, T. J.; Li, Z.*; Catalytic One-Pot Conversion of Renewable Platform Chemicals to Hydrocarbon and Ether Biofuels via Tandem Hf(OTf)4 + Pd/C Catalysis, ChemSusChem 2019, 12, 5217-5223. (Cover Feature: ChemSusChem 2019, 12, 5214.) Liu, H.; Zhu, Y.-L.; Li, Z.*; Catalytic amidation of natural and synthetic polyol esters with sulfonamides, Nat. Commun. 2019, 10, 3881. (Open access. 自然科学基金委科学传播中心: http://www.nsfc.gov.cn/csc/20340/20343/45548/; 科学网: http://news.sciencenet.cn/sbhtmlnews/2019/10/350000.shtm) Xu, X.-L.; Li, Z.*; Catalytic Redox Chain Ring Opening of Lactones with Quinones To Synthesize Quinone-Containing Carboxylic Acids; Org. Lett., 2019, 21, 5078-5081. (CBG: https://www.chembeango.com/zixun/43665) Xu, X.-L.; Li, Z.*; Deciphering the Redox Chain Mechanism in the Catalytic Alkylation of Quinones; Synlett, 2018, 29, 1807-1813. (Invited Synpact article) Xu, X.-L.; Li, Z.* Catalytic Electrophilic Alkylation of p-Quinones through a Redox Chain Reaction; Angew. Chem. Int. Ed., 2017, 56, 8196–8200. (Top 3 Most Accessed Communications of June 2017; X-MOL: http://www.x-mol.com/news/8483)
PhD & Postdoc publications: 1. Lohr, T. L.; Li, Z.; Marks, T. J.* Thermodynamic Strategies for C–O Bond Formation and Cleavage via Tandem Catalysis; Acc. Chem. Res., 2016, 49 (5), pp 824–834. 2. Lohr, T. L.; Li, Z.; Assary, R. S.; Curtiss, L. A.; Marks, T. J.* Mono- and tri-ester hydrogenolysis using tandem catalysis. Scope and mechanism; Energy Environ. Sci., 2016, 9 (2), pp 550–564. 3. Lohr, T. L.; Li, Z.; Marks, T. J.* Selective Ether/Ester C–O Cleavage of an Acetylated Lignin Model via Tandem Catalysis; ACS Catal., 2015, 5 (11), pp 7004–7007. 4. Lohr, T. L.†; Li, Z.†; Assary, R. S.; Curtiss, L. A.; Marks, T. J.* Thermodynamically Leveraged Tandem Catalysis for Ester RC(O)O–R′ Bond Hydrogenolysis. Scope and Mechanism; ACS Catal.,2015, 5 (6), pp 3675–3679. (Equal contribution) 5. Li, Z.; Assary, R. S.; Atesin, A. C.; Curtiss, L. A.; Marks, T. J.* Rapid Ether and Alcohol C-O Bond Hydrogenolysis Catalyzed by Tandem High-valent Metal Triflate + Supported Pd Catalysts; J. Am. Chem. Soc., 2014, 136, 104-107. 6. Assary, R. S.*; Atesin, A. C.; Li, Z.; Curtiss, L. A.*; Marks, T. J.* Reaction Pathways and Energetics of Etheric C–O Bond Cleavage Catalyzed by Lanthanide Triflates; ACS Catal., 2013, 3, 1908–1914. 7. Olivares-Romero, J. L.; Li, Z.; Yamamoto, H.* Catalytic Enantioselective Epoxidation of Tertiary Allylic and Homoallylic alcohols; J. Am. Chem. Soc., 2013, 135, 3411-3413. 8. Li, Z.; Yamamoto, H.* Hydroxamic Acids in Asymmetric Synthesis; Acc. Chem. Res.,2013,46, 506-518. 9. Olivares-Romero, J. L.; Li, Z.; Yamamoto, H.*; Hf(IV)-Catalyzed Enantioselective Epoxidation of N-Alkenyl Sulfonamides and N-Tosyl Imines; J. Am. Chem. Soc., 2012, 134, 5440-5443. (Top 20 Most Read Articles of March 2012) 10. Li, Z.; Yamamoto, H.*; Zirconium(IV)- and Hafnium(IV)-Catalyzed Highly Enantioselective Epoxidation of Homoallylic and Bishomoallylic Alcohols; J. Am. Chem. Soc. 2010, 132, 7878-7880. 11. Li, Z.; Zhang, W.; Yamamoto, H.*; Vanadium-Catalyzed Enantioselective Desymmetrization of meso-Secondary Allylic Alcohols and Homoallylic Alcohols; Angew. Chem. Int. Ed., 2008, 47, 7520-7522; Angew. Chem., 2008,120, 7630-7632. |