Recently, School of Physical Science and Technology (SPST) Assistant Professor Lin Bolin’s research group made a major breakthrough in CO2/olefins-based polymeric materials synthesis and was selected in ACS Editors’ Choice. Combining green-house gas CO2 with a low cost olefin, they provided a convenient and highly efficient methodology for synthesis of functionalizable polymeric materials. This efficient method not only solves a crucial problem for the potential application of CO2/olefin-derived polymer materials in industry, but also extends new strategies for CO2 transformation and utilization. This research progress was published as Highly Efficient Synthesis of Functionalizable Polymers from a CO2/1,3-Butadiene-DerivedLactone in ACS Macro Letters and was also selected in ACS Editors’ Choice. In addition, part of their work was won Excellent PosterAward twice at the 15th International Conference on Carbon Dioxide Utilization (ICCDU XV) in July and the in National Polymer Research Seminar in October.
Polymeric materials based on petroleum industry are one of the most important material foundations of modern socio-economy. Five of the current “big six” synthetic polymers are made from olefins via C-C bond formations. Developing sustainable alternatives represents a major challenge in current polymer industry. CO2 is a highly attractive renewable C1 chemical feedstock, due to its abundance, availability, recyclability, and non-toxicity. Making polymers from CO2 , especially copolymerization of CO2 and olefins, has been long sought by numerous polymer chemists.As one of the important components in the C4 fraction of oil cracking, 1,3-butadiene can also be derived from succinic acid, which is ranked as one of the top 12 biomass platform compounds best suited to replace petrochemicals. Thus, chemistry leading to polymers made from CO2 and large-volume dienes, especially 1,3-butadiene, is of significant fundamental interest and promising large-scale industrial utility. 1,3-Butadiene could react with CO2 to produce a lactone efficiently via C-C bond coupling. Polymerization of the lactone has long been considered as one of the most promising avenues to make copolymers from CO2 and olefins. Though researchers tried the polymerization of the lactone about twenty years ago, only one success has been achieved with relatively low conversions under the assistance of metal additives and complex radical initiators. Lin’s research group developed an additive/solvent-free bulk polymerization method, leading to a quantitative monomer conversion (up to 100%) and polymers with molar mass up to 239 kDa as well as high contents of CO2 (29 wt %) and preserved functional olefins of the obtained polymer (poly-L). Through detailed mechanistic studies, it was found that oxygen, commonly considered as a radical inhibitor, acted as an initiator in the system. Possible initiation mechanisms were proposed based on the unique polymer structure.
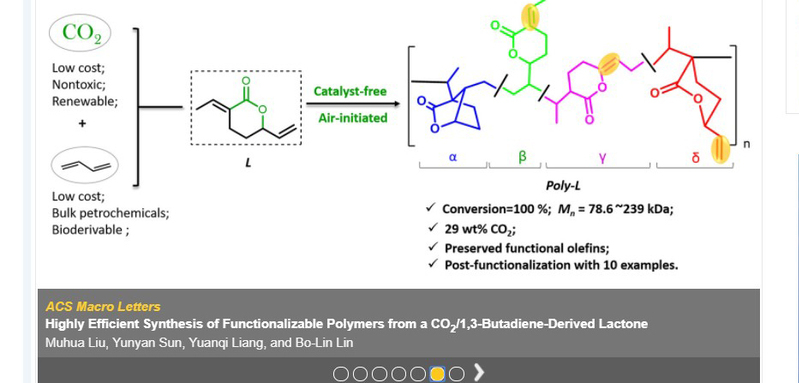
It is worth mentioning that postmodifications of poly-L can be readily achieved by taking advantage of the abundant C=C bonds in the polymeric structures. This work also demonstrates the potentials of poly-L’s functionalizations by uitilizing the thiol-ene click chemistry. All thiols involved in the modification showed moderate reactivity. The success of coupling the poly-L with cysteine suggested its great potential for biological applications.
ACS Editors' Choice®is a new service which features scientific articles of broad public interest and makes this material widely accessible to both the scholarly community and general public. Starting January 2014, ACS selects, features, and makes openly available one article per day to highlight the newly published work of an author and research team. Articles selected with less than 1% ratio will be based on timely recommendations from the more than 400 Editors of ACS's 51 peer-reviewed journals. Under ACS Editors‘ Choice, the articles are free open access by ACS due to its potential for broad public interest, an honor given to only one article from the entire ACS portfolio each day of the year. Professor Peter J. Stang, chief editor of Journal of American Chemical Society, pointed out that ideally each article recommended by editors of ACS publications should contain new concepts that are paradigm-shifting in the field.
Paper Link: http://pubs.acs.org/doi/full/10.1021/acsmacrolett.7b00774


